Mentre impazza la corsa ai vaccini e i governi le stanno mettendo in campo di tutti i colori per introdurre un illegittimo obbligo, più seriamente l’autorevole ‘British Medical Journal’pubblica un lungo articolo che illustra l’utilità delle cure per fronteggiare il Covid-19.
Parliamo di quelle cure, di quei farmaci fin dall’inizio osteggiati, boicottati e delegittimati con tutti i mezzi possibili dai governi di vari paesi europei. In primo luogo il nostro, che attraverso il suo ministro per la Salute Roberto Speranza ha indicato agli italiani una unica e sola via in attesa messianica dei vaccini: “tachipirina e vigile attesa”, questo il folle percorso terapeutico indicato. E guai a quei medici che osavano prescrivere quelle cure, quei farmaci fuorilegge. Come, per fare i due esempi più clamorosi, l’Idrossiclorochina el’Invermectina.
C’è voluta addirittura un’ordinanza del Consiglio di Stato, varata il 12 dicembre 2020, per sdoganare l’uso dell’idrossiclorochina, e far in modo che i medici di famiglia me potessero prescrivere l’uso come validissima cura d’urto contro il Covid incipiente.
Un farmaco che ha anche un altro grosso difetto, o meglio due: è in commercio da anni (viene ritualmente usato contro l’artrosi) e quindi è reperibile in tutte le farmacie; e, soprattutto, è molto economico, quindi non consente grossi utili alle case farmaceutiche. Quelle aziende impegnate, invece, nella stramiliardaria corsa ai vaccini, che – lo ribadiamo per l’ennesima volta – sono del tutto sperimentali, perché i test base termineranno solo a dicembre 2013, quindi fra due anni e mezzo. Per adesso le cavie siamo noi.
E’ stato (ed è ancora) quindi delittuoso negare l’accesso ai farmaci anti covid: di tale reato (strage) dovranno rispondere le autorità scientifiche (sic) e politiche che hanno impedito le cure e costretto migliaia e migliaia di cittadini ai ricoveri, alle drammatiche intubazioni e, in moltissimi casi, alla morte, come tragicamente mostrano le nostre ‘performance’ (sic) a livello europeo.
Ecco, di seguito, cosa scrive il ‘British Medical Journal’. Il testo, ovviamente, è in inglese.
A living WHO guideline on drugs for covid-19
Abstract
Clinical question What is the role of drug interventions in the treatment of patients with covid-19?
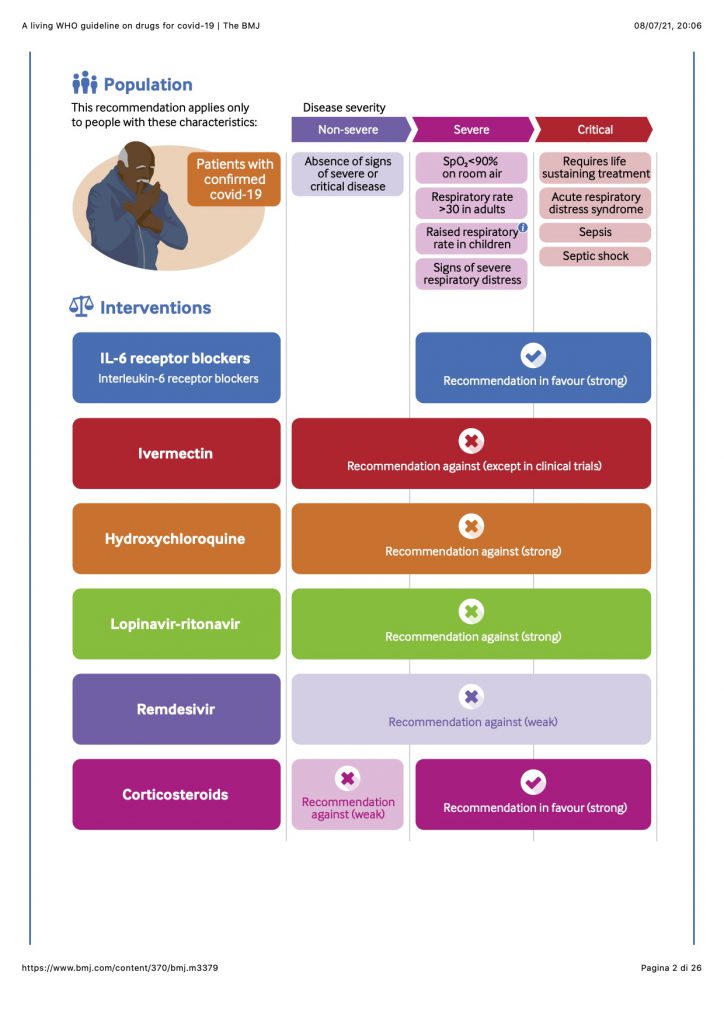
New recommendation The publication of the RECOVERY and REMAP-CAP randomised controlled trials triggered this guideline update, resulting in a strong recommendation for interleukin-6 (IL-6) receptor blockers (tocilizumab or sarilumab) in patients with severe or critical covid-19.
Prior recommendations (a) A recommendation not to use ivermectin in patients with covid-19, regardless of disease severity, except in the context of a clinical trial; (b) a strong recommendation against the use of hydroxychloroquine in patients with covid-19, regardless of disease severity; (c) a strong recommendation against the use of lopinavir-ritonavir in patients with covid-19, regardless of disease severity; (d) a strong recommendation for systemic corticosteroids in patients with severe and critical covid-19; (e) a conditional recommendation against systemic corticosteroids in patients with non-severe covid-19; and (f) a conditional recommendation against remdesivir in hospitalised patients with covid-19.
How this guideline was created This living guideline is from the World Health Organization (WHO) and provides up to date covid-19 guidance to inform policy and practice worldwide. Magic Evidence Ecosystem Foundation (MAGIC) provided methodological support. A living systematic review with network meta-analysis informed the recommendations. For IL-6 receptor blockers, a complementary prospective meta-analysis informed the outcome of mortality. An international guideline development panel of content experts, clinicians, patients, an ethicist and methodologists produced recommendations following standards for trustworthy guideline development using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Understanding the new recommendation The panel judged that almost all well informed patients would want to receive IL-6 receptor blockers (tocilizumab and sarilumab) for severe and critical covid-19, given that there was high certainty evidence of benefit for mortality and mechanical ventilation, the two most important outcomes for patients. In addition to the network analysis, a direct comparison from REMAP-CAP provided evidence that tocilizumab and sarilumab had similar effects on the main outcomes of interest. There is ongoing uncertainty about serious adverse events and bacterial infections related to IL-6 receptor blockers. The panel recognised important resources and access issues around IL-6 receptor blockers.
Updates This is a living guideline. It replaces earlier versions (4 September, 20 November, 17 December 2020, and 31 March 2021) and supersedes the BMJ Rapid Recommendations on remdesivir published on 2 July 2020. The previous versions can be found as data supplements. New recommendations will be published as updates to this guideline.
Readers note This is the fifth version (update 4) of the living guideline (BMJ 2020;370:m3379). When citing this article, please consider adding the update number and date of access for clarity.
This living guideline responds to emerging evidence from randomised controlled trials (RCTs) on existing and new drug treatments for covid-19. Although case numbers are falling in some regions, they are rising in others. Vaccines are linked to falling case numbers and hospitalisations, but it is unclear how long protection following vaccination or natural infection will last, or how this might alter with the emergence of new variants. Therefore, the potential for drugs to treat people infected with covid-19 remains of interest and is the focus of this guideline. A linked guideline addresses the role of drugs in the prevention of covid-19 among people who are not infected.1
More than 3800 trials on covid-19 interventions have been registered or are ongoing (see section on emerging evidence2). Among these are large national and international platform trials (such as RECOVERY, WHO SOLIDARITY, REMAP-CAP, and ACTIV) that recruit large numbers of patients in many countries, with pragmatic and adaptive designs.3456 These platform trials are currently investigating and reporting on numerous interventions, including antiviral monoclonal antibodies and immunomodulators. This rapidly evolving evidence landscape requires trustworthy interpretation and expeditious clinical practice guidelines to inform clinicians and healthcare decision makers.
A living network meta-analysis associated with this guideline will incorporate emerging trial data and allows for analysis of comparative effectiveness of multiple covid-19 treatments.7 This network meta-analysis and other related publications are included in box 1. We also use additional relevant evidence on safety, prognosis, and patient values and preferences related to covid-19 treatments to inform the living guidance.
Linked resources in this BMJ Rapid Recommendations cluster
-
Rochwerg B, Agarwal A, Siemieniuk RAC, et al. A living WHO guideline on drugs for covid-19 [Update 4]. BMJ2020;370:m3379, doi:10.1136/bmj.m3379
-
World Health Organization. Therapeutics and COVID-19. Living guideline. June 2021. https://www.who.int/publications/i/item/therapeutics-and-covid-19-living-guideline.
-
MAGICapp (https://app.magicapp.org/#/guideline/nBkO1E)
-
Expanded version of the methods, processes, and results with multilayered recommendations, evidence summaries, and decision aids for use on all devices
-
-
Siemieniuk RAC, Bartoszko JJ, Ge L, et al. Drug treatments for covid-19: living systematic review and network meta-analysis [Update 4]. BMJ 2020;370:m2980, doi:10.1136/bmj.m2980
-
Zeraatkar D, Cusano E, Diaz Martinez JP, et al. Tocilizumab and sarilumab alone or in combination with corticosteroids for COVID-19: a systematic review and network meta-analysis. medRxiv (forthcoming)
-
Izcovich A, Siemieniuk RAC, Bartoszko JJ, et al. Adverse effects of remdesivir, hydroxychloroquine, and lopinavir/ritonavir when used for COVID-19: systematic review and meta-analysis of randomized trials. medRxiv 2020 https://www.medrxiv.org/content/10.1101/2020.11.16.20232876v1
What triggered this version of the guideline?
This is the fifth version of this guideline, and it addresses the use of interleukin-6 (IL-6) receptor blockers in patients with severe and critical covid-19. It was triggered by the publications from the RECOVERY and REMAP-CAP platform trials, which suggested benefit of this class of drugs. It was finalised when data from the direct comparison between tocilizumab and sarilumab from REMAP-CAP became available to WHO.8
How to use this guideline
This is a living guideline, so the recommendations included here will be updated and new recommendations will be added for other drugs for covid-19. The infographic provides a summary of the recommendations and includes links to the MAGICapp for more details on the evidence and rationale for the recommendation, as well as patient decision aids. Box 2 outlines key methodological aspects of the guideline process.
How this living guideline was created (see MAGICapp for full details https://app.magicapp.org/#/guideline/nBkO1E)
This guideline was developed by WHO and the MAGIC Evidence Ecosystem Foundation (MAGIC), with support from The BMJ. It is driven by an urgent need for trustworthy and living guidance to rapidly inform policy and practice worldwide during the covid-19 pandemic. WHO has partnered with MAGIC for their methodologic support in the development and dissemination of living guidance for covid-19 drug treatments, in the form of BMJ Rapid Recommendations, to provide patients, clinicians, and policy makers with up to date, evidence based, and user friendly guidelines.
Standards, methods, and processes for living and trustworthy guidance
The panel produced the recommendations following standards for trustworthy guideline development using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach, in compliance with the WHO Handbook for Guideline Development 2nd Edition,9 the Institute of Medicine, and the Guideline International Network (G-I-N).10 Details are provided in the WHO guideline (https://www.who.int/publications/i/item/therapeutics-and-covid-19-living-guideline) and MAGICapp (https://app.magicapp.org/#/guideline/nBkO1E).
Selection and support of the panel
For the IL-6 receptor blocker recommendation, WHO convened an international guideline development panel with 34 individuals, of whom 28 were content experts (clinicians, methodologists, scientists) and four were patients who had survived covid-19. The methods chair (methodological expertise) and a clinical chair (content expertise) guided the panel discussions. Panel members were invited by WHO, after consultation with the methods chair and MAGIC, with the aim of achieving gender, geography, expertise, and patient representation balance in the panel. No relevant conflict of interest was identified for any panel member.
As recommended by the WHO handbook, the guideline development panel aimed to create a recommendation based on consensus but elected, at the beginning of the first panel meeting, to call a vote if a consensus could not be reached. These procedures proved unnecessary for this recommendation.
Guideline perspective, outcomes, and values and preferences
The target audience for this guidance consists primarily of clinicians, but secondarily of patients and healthcare decision makers. The panel considered an individual patient perspective but also took account of contextual factors (such as resources, feasibility, acceptability, equity) to accommodate global re-use and adaptation for countries and healthcare systems.
During a pandemic, access to healthcare may vary over time and between different countries. The panel defined covid-19 by clinical severity, and mutually exclusive definitions are provided in box 3.
There were insufficient published data to provide the guideline development panel with an informative systematic review of studies describing patients’ experiences or values and preferences on treatment decisions for covid-19 drug treatments. The panel therefore relied on their own judgments of what well informed patients would value after carefully balancing the benefits, harms, and burdens of treatment and their subsequent treatment preferences. The panel included four patient representatives who had lived experience with covid-19.
The panel agreed that the following values and preferences would be representative of those of typical well informed patients:
-
Most patients would be reluctant to use a medication for which the evidence left high uncertainty regarding effects on the outcomes they consider important. This was particularly so when evidence suggested treatment effects, if they exist, are small and the possibility of important harm remains.
-
In an alternative situation with larger benefits and less uncertainty regarding both benefits and harms, more patients would be inclined to choose the intervention.
Although the panel focused on an individual patient perspective, they also considered a population perspective in which feasibility, acceptability, equity, and cost are important considerations.
Sources of evidence
To create recommendations, the panel relied on evidence synthesised in a living network meta-analysis led by MAGIC.7 The panel also considered data from a WHO-sponsored prospective meta-analysis which included some previously unpublished data evaluating sarilumab that was subsequently included in the network meta-analysis.11 This prospective meta-analysis was used to inform the outcome of mortality. While the investigators responsible for the meta-analyses rated the certainty of the evidence, this was re-assessed independently by the guideline panel.
Derivation of absolute effects for drug treatments
The control arm of the WHO SOLIDARITY trial, performed across a wide variety of countries and geographical regions, was identified by the guideline panel as generally representing the most relevant source of evidence for baseline risk estimates for mortality and mechanical ventilation.5 The rationale for selecting the WHO SOLIDARITY trial was to reflect the overall prognosis of the global population for which the WHO guideline recommendations are made. When applying the evidence to a particular patient or setting, for any medication with a convincing effect, clinicians should consider the individual’s risk of mortality and need for mechanical ventilation. In view of the study designs, the panel determined that, for other outcomes, using the median or mean of all patients randomised to usual care across the included studies would provide the most reliable estimate of baseline risk.711
Systemic corticosteroids now represent standard of care in patients with severe and critical covid-19 (see strong recommendation issued by WHO September 2020). Therefore, the baseline risk estimates in the IL-6 receptor blocker evidence summaries were adjusted for treatment effects of corticosteroids for the outcome of mortality and mechanical ventilation. This warranted an update of the evidence summaries for corticosteroids, with SOLIDARITY replacing the original UK cohort study informing the initial (and considerably higher) baseline risk estimates for mortality.5
Of note, baseline risks, and thus absolute effects, may vary significantly geographically and over time. As such, users of this guideline may prefer estimating absolute effects by using local event rates.
Who do the recommendations apply to?
This guideline applies to all patients with covid-19. For some drugs, recommendations may differ based on the severity of covid-19 disease. The guideline development panel elected to use the WHO severity definitions based on clinical indicators, adapted from WHO covid-19 severity categorisation (see box 3).12 These definitions avoid reliance on access to healthcare to define patient subgroups. The infographic illustrates these three disease severity groups and key characteristics to apply in practice.
WHO definitions of disease severity for covid-19
-
Critical covid-19—Defined by the criteria for acute respiratory distress syndrome (ARDS), sepsis, septic shock, or other conditions that would normally require the provision of life sustaining therapies such as mechanical ventilation (invasive or non-invasive) or vasopressor therapy.
-
Severe covid-19—Defined by any of:
-
Oxygen saturation <90% on room air*
-
Respiratory rate >30 breaths per minute in adults and children >5 years old, ≥60 breaths/min in children <2 months old, ≥50 in children 2-11 months old, and ≥40 in children 1-5 years old
-
Signs of severe respiratory distress (accessory muscle use, inability to complete full sentences, and, in children, very severe chest wall indrawing, grunting, central cyanosis, or presence of any other general danger signs).
-
-
Non-severe covid-19—Defined as absence of any signs of severe or critical covid-19.
-
*The guideline development panel noted that the oxygen saturation threshold of 90% to define severe covid-19 was arbitrary and should be interpreted cautiously when defining disease severity. For example, clinicians must use their judgment to determine whether a low oxygen saturation is a sign of severity or is normal for a given patient with chronic lung disease. Similarly, a saturation of 90-94% is abnormal, and can be an early sign of severe disease, if the patient is on a downward trend. Generally, if there is any doubt, the panel suggested erring on the side of considering the illness as severe.
The guidance
Interleukin-6 receptor blockers
IL-6 receptor blockers tocilizumab and sarilumab are monoclonal antibodies approved for use in rheumatoid arthritis. Elevated IL-6 concentrations are associated with severe outcomes in covid-19, including respiratory failure and death. IL-6 receptor blockers antagonise membrane-bound and soluble forms of the IL-6 receptor, blocking the cytokine’s activation and regulation of the immune response to infection.
Evidence underpinning the recommendation is outlined in box 4. In addition to the RECOVERY and REMAP-CAP trial publications from February 2021,1314 new trial data from 1020 patients directly comparing tociluzimab and sarilumab in REMAP-CAP were made available to the WHO on 1 June 2021.8 This new evidence, synthesised through the living network meta-analysis and the prospective meta-analysis,711 increased certainty that tocilizumab and sarilumab are similar in their effects on outcomes of interest.
Interleukin-6 (IL-6) receptor blocker trial data
The living network meta-analysis pooled data from 30 RCTs with 10 618 participants, and these data were used by the guideline development panel for all outcomes other than mortality (appendix 5).7 All trials included patients with severe or critical covid-19: 37% were published in peer reviewed journals, 3% were available as preprints, and 60% were completed but unpublished. We used the prospective meta-analysis for mortality, because it included additional, unpublished data. The prospective meta-analysis pooled data from 22 RCTs with 10 156 participants.11
Pooled estimates demonstrated a reduction in mortality and need for invasive mechanical ventilation, and possible reduction in duration of both mechanical ventilation and hospitalisation with IL-6 receptor blockers. The evidence regarding the risk of serious adverse events is uncertain. Low certainty evidence suggested that the risk of bacterial infections in the context of immunomodulatory treatment with IL-6 receptor blockers may be similar to usual care.
Subgroup analysis
The panel determined that there was no subgroup effect across all pre-specified outcomes of interest based on disease severity (severe versus critical disease). There were insufficient data to assess subgroup effect by elevation of inflammatory markers or age. The panel considered the results of a subgroup analysis by baseline systemic corticosteroid use for the outcome of mortality. This suggested that the relative effects of IL-6 receptor blockers may vary as a function of the use of systemic corticosteroids at baseline. Crucially, steroids did not abolish and may even enhance the beneficial effect of IL-6 receptor blockers on mortality. For reasons described below, the panel did not formally evaluate the credibility of this subgroup analysis.
When comparing tocilizumab and sarilumab, based on the prospective meta-analysis, there was no evidence of a subgroup effect. However, there were more data and therefore greater precision for tocilizumab+steroids versus steroids alone (odds ratio 0.76, 95% confidence interval 0.67 to 0.87) compared with sarilumab+steroids versus steroids alone (odds ratio 0.91, 0.59 to 1.41). This is evident as the upper limit of the 95% confidence interval does not cross no effect with tocilizumab, but does with sarilumab. In addition to these subgroup data, the guideline panel reviewed direct comparison data from REMAP-CAP investigators, which demonstrated no difference between tocilizumab and sarilumab in a population of patients all receiving corticosteroids (36.5% mortality with tocilizumab, 33.9% mortality with sarilumab). The network meta-analysis estimate of tocilizumab+steroids versus sarilumab+steroids, incorporating both direct and indirect data, provided moderate certainty data of no difference between drugs (odds ratio 1.07, 0.86 to 1.34).7 Therefore, considering the totality of the data, the panel decided to make the same strong recommendation for both drugs.
Understanding the recommendation on IL-6 receptor blockers
We recommend treatment with IL-6 receptor blockers (tocilizumab or sarilumab) for patients with severe or critical covid-19. (Note: corticosteroids have previously been strongly recommended in patients with severe and critical covid-19, and we recommend patients meeting these severity criteria should now receive both corticosteroids and IL-6 receptor blockers.)
Balance of benefit and harm—There was high certainty evidence for a clinically important reduction in mortality and need for mechanical ventilation. The effects of IL-6 receptor blockers on duration of both hospitalisation and mechanical ventilation are uncertain (low certainty evidence; serious risk of bias due to lack of blinding and serious inconsistency) (box 4).
There was uncertainty about the risk of serious adverse effects (low certainty evidence). The risk of bacterial infections with immunomodulatory IL-6 receptor blocker therapy may be similar to usual care. However, the guideline development panel had some concerns that, given the short term follow-up of most trials and the challenges associated with accurately capturing adverse events such as bacterial or fungal infection, the evidence summary may under-represent the risks of treatment with IL-6 receptor blockers. Furthermore, the trials of IL-6 receptor blockers that inform this recommendation were mostly performed in high income countries where the risk of infectious complications may be less than in some other parts of the world, and so the generalisability of the data on these adverse events is unclear.
Values and preferences—The guideline panel inferred that almost all well informed patients with severe or critical covid-19 infection would want to receive IL-6 receptor blockers given the reduction in mortality and mechanical ventilation, despite the low certainty around serious adverse events. A minority of the panel felt that a significant proportion of patients might decline the intervention due to the uncertainties around harms and taking account of the small reduction in mortality.
Resource implications, feasibility, equity, and human rights—IL-6 receptor blockers require intravenous administration but only require one, or at most two, doses.
Compared with other treatments for covid-19, IL-6 receptor blockers are expensive. The recommendation does not take account of cost effectiveness. Access to these drugs is challenging in many parts of the world, and this recommendation could exacerbate health inequity. However, this strong recommendation should provide a stimulus to improve global access to these treatments.
At a time of drug shortage, many jurisdictions have suggested triaging use of IL-6 receptor blockers. Strategies for this include prioritising patients with the highest baseline risk for mortality (those with critical disease over those with severe disease), in whom the absolute benefit of treatment is therefore greatest. The relative effects (odds ratio 0.87) for reduction in mortality with IL-6 receptor blockers result in 28 fewer deaths per 1000 (95% confidence interval 9 to 47 fewer deaths) in critically ill patients compared with 12 fewer deaths per 1000 (4 to 19 fewer deaths) in the severely ill.
Other suggestions, which lack direct evidence, include prioritising patients who are deteriorating despite corticosteroid treatment and avoiding use in those with established multi-organ failure (in whom the benefit is likely to be smaller). Finally, sarilumab is not indicated to be used in children, and therefore there could be a preference for tocilizumab in this subgroup.
Ivermectin (published 31 March 2021)
The recommendation addressing ivermectin was informed by results from a systematic review and network meta-analysis that pooled data from 16 RCTs with 2407 participants. Of the included trials, 75% examined patients with non-severe disease and 25% included both severe and non-severe patients. None of the included RCTs enrolled children under 15 years old or pregnant women. Given this, the applicability of this recommendation to children is uncertain, though there is no rationale to suggest they would respond differently.
Understanding the recommendation on ivermectin
We recommend not to use ivermectin in patients with covid-19 except in the context of a clinical trial, regardless of disease severity or duration of symptoms.
Balance of benefit and harm—For most important outcomes, the guideline panel considered the evidence to be of very low certainty. A combination of serious risk of bias and very serious imprecision contributed to very low certainty of evidence for mortality, despite a point estimate and confidence interval that seem to suggest benefit with ivermectin. The picture was similar for other important outcomes, including mechanical ventilation, hospital admission, duration of hospitalisation, and viral clearance. The very low certainty of evidence was a critical factor in the recommendation.
Ivermectin may have little or no effect on time to clinical improvement (low certainty evidence) and may increase the risk of adverse effects leading to drug discontinuation (low certainty evidence). A recommendation to only use a drug in the setting of a clinical trials is appropriate when there is very low certainty evidence and future research has a large potential for reducing uncertainty about the effects of the intervention and for doing so at reasonable cost.
Subgroup analyses indicated no effect modification based on dose. We were unable to examine subgroups based on patient age or severity of illness due to insufficient trial data. Therefore, we assumed similar effects in all subgroups.
Values and preferences—The guideline panel inferred that almost all well informed patients would not want to receive ivermectin, given the evidence left a very high degree of uncertainty in effect on critical outcomes and there was a possibility of harms, such as adverse events associated with treatment (box 2). The panel did not expect there would be much variation among patients in values and preferences when it came to this intervention.
Resource implications, feasibility, equity, and human rights—Although the cost of ivermectin may be low per patient, the panel raised concerns about diverting attention and resources away from care likely to provide a benefit such as corticosteroids in patients with severe covid-19 and other supportive care interventions. Also, use of ivermectin for covid-19 would divert supply away from pathologies for which it is clearly indicated, potentially contributing to drug shortages, especially for helminth control and elimination programmes. If corticosteroids are used in the treatment of covid-19, empiric treatment with ivermectin may still be considered in strongyloidiasis-endemic areas, albeit not for treatment of covid-19 itself.
Hydroxychloroquine (published 17 December 2020)
The recommendation addressing hydroxychloroquine was informed by results from the same systematic review and network meta-analysis that pooled data from 30 RCTs with 10 921 participants. Of note, none of the included RCTs enrolled children or adolescents under the age of 19 years. Given this, the applicability of this recommendation to children is currently uncertain.
Understanding the recommendation on hydroxychloroquine
We recommend against using hydroxychloroquine or chloroquine in addition to usual care for the treatment of patients with covid-19, regardless of disease severity or duration of symptoms (strong recommendation).
Balance of benefit and harm—Hydroxychloroquine and chloroquine probably do not reduce mortality or mechanical ventilation and may not reduce duration of hospitalisation. The evidence does not exclude the potential for a small increased risk of death and mechanical ventilation with hydroxychloroquine. The effect on other less important outcomes—including time to symptom resolution, admission to hospital, and duration of mechanical ventilation—remains uncertain.
Hydroxychloroquine may increase the risk of diarrhoea and nausea or vomiting, a finding consistent with evidence from its use in other conditions. Diarrhoea and vomiting may increase the risk of hypovolaemia, hypotension, and acute kidney injury, especially in settings where healthcare resources are limited. Whether and to what degree hydroxychloroquine increases the risk of cardiac toxicity, including life-threatening arrhythmias, when used in patients with covid-19 is uncertain.
Subgroup analyses indicated no effect modification based on severity of illness (comparing either critical versus severe/non-severe or non-severe versus critical/severe) or age (comparing those aged <70 years versus those ≥70 years). Further, the cumulative dose and predicted day 3 serum trough concentrations (lowest predicted blood concentration on day 3) did not modify the effect for any outcome. Therefore, we assumed similar effects in all subgroups.
We also reviewed evidence comparing the use of hydroxychloroquine plus azithromycin versus hydroxychloroquine alone. There was no evidence that the addition of azithromycin modified the effect of hydroxychloroquine for any outcome (very low certainty).
Values and preferences—Applying the agreed values and preferences (box 2), the guideline +6panel inferred that almost all well informed patients would not want to receive hydroxychloroquine given the evidence suggesting there was probably no effect on mortality or need for mechanical ventilation and that there was a risk of adverse events including diarrhoea and nausea/vomiting. The panel did not expect there would be much variation in values and preferences among patients when it came to this intervention.
Resource implications, feasibility, equity, and human rights—Hydroxychloroquine and chloroquine are relatively inexpensive compared with other drugs used for covid-19 and are already widely available, including in low income settings. Despite this, the panel felt that almost all patients would choose not to use hydroxychloroquine or chloroquine because the harms outweigh the benefits. Although the cost may be low per patient, the panel raised concerns about diverting attention and resources away from care likely to provide a benefit such as corticosteroids in patients with severe covid-19 and other supportive care interventions.
Lopinavir-ritonavir (published 17 December 2020)
The recommendation addressing lopinavir-ritonavir was informed by the same systematic review and network meta-analysis, including data from seven RCTs with 7429 participants. None of the included RCTs enrolled children or adolescents under the age of 19 years, so the applicability of this recommendation to children is uncertain.
Understanding the recommendation on lopinavir-ritonavir
We recommend against using lopinavir-ritonavir in addition to usual care for the treatment of patients with covid-19, regardless of disease severity and duration of symptoms (strong recommendation).
Balance of benefit and harm—The guideline panel found a lack of evidence that lopinavir-ritonavir improved patient-important outcomes such as reduced mortality, need for mechanical ventilation, time to clinical improvement, and others. For mortality and need for mechanical ventilation, this was based on moderate certainty evidence; for the other outcomes, this was based on low or very low certainty evidence.
There was low certainty evidence that lopinavir-ritonavir may increase the risk of diarrhoea and nausea or vomiting, a finding consistent with the indirect evidence evaluating its use in patients with HIV infection. Diarrhoea and vomiting may increase the risk of hypovolaemia, hypotension, and acute kidney injury, especially in settings where healthcare resources are limited. There was an uncertain effect on viral clearance and acute kidney injury.
Subgroup analysis indicated no effect modification based on severity of illness (comparing either critical versus severe/non-severe or non-severe versus critical/severe) or age (comparing those aged <70 years versus those ≥70 years). As there was no evidence of a statistical subgroup effect, we did not formally evaluate credibility. Although the trials did not report subgroup effects by time from symptom onset, many of the trials enrolled patients early in the disease course. The guideline panel therefore felt that the evidence applies to all patients with covid-19.
Values and preferences—Applying the agreed values and preferences (box 2), the guideline panel inferred that almost all well informed patients would not want to receive lopinavir-ritonavir given that the evidence suggested there was probably no effect on mortality or need for mechanical ventilation and there was a risk of adverse events including diarrhoea and nausea or vomiting. The panel did not expect there would be much variation in values and preferences between patients for this intervention.
Resource implications, feasibility, equity, and human rights—Although the cost of lopinavir-ritonavir is not as high as some other investigational drugs for covid-19 and the drug is generally available in most healthcare settings, the panel raised concerns about opportunity costs and the importance of not drawing attention and resources away from best supportive care or the use of corticosteroids in severe covid-19.
Remdesivir (published 20 November 2020)
The recommendation addressing remdesivir was informed by the same systematic review and network meta-analysis, including data from four RCTs with 7333 participants hospitalised for covid-19. Of note, none of the included RCTs enrolled children or adolescents under the age of 19 years, and, although older people were included in the trials, their outcomes were not reported separately. Also, there is no pharmacokinetic or safety data on remdesivir for children. Given this, the applicability of this recommendation to children is currently uncertain.
Understanding the recommendation on remdesivir
We suggest against administering remdesivir in addition to usual care for the treatment of patients hospitalised with covid-19, regardless of disease severity (weak or conditional recommendation).
When moving from evidence to the conditional recommendation against the use of remdesivir for patients with covid-19, the panel emphasised the evidence of possibly no effect on mortality, need for mechanical ventilation, time to clinical improvement, and other patient-important outcomes, albeit of low certainty; it also noted the anticipated variability in patient values and preferences and other contextual factors, such as resource considerations, accessibility, feasibility and impact on health equity (see below).
Balance of benefit and harm—The guideline panel found a lack of evidence that remdesivir improved outcomes that matter to patients such as reduced mortality, need for mechanical ventilation, time to clinical improvement, and others. However, the low certainty evidence for these outcomes, especially mortality, does not prove that remdesivir is ineffective; rather, there is insufficient evidence to confirm that it does improve patient-important outcomes.
There was no evidence of increased risk of serious adverse events in patients receiving remdesivir, at least from the included trials. Further pharmacovigilance is required, because serious adverse events are commonly underreported and rare events could be missed, even in large RCTs.
Data from the network meta-analysis indicated that a subgroup of people with non-critical disease might benefit from remdesivir. However, the panel judged the credibility in this subgroup analysis to be insufficient to make subgroup recommendations.15 Important factors influencing this decision included a lack of a priori hypothesised direction of subgroup effect by trial investigators, little or no previously existing supportive evidence for the subgroup finding, and relatively arbitrary cut points used to examine the subgroups of interest. The overall low certainty evidence for the benefits and harms of remdesivir, driven by risk of bias and imprecision limitations, also contributed to the judgment (see WHO guidance and MAGICapp linked from box 1 for full details). The panel highlighted that, despite the conditional recommendation against remdesivir, they support further enrolment into RCTs evaluating remdesivir, especially to provide higher certainty of evidence for specific subgroups of patients. The panel had a priori requested analyses of other important subgroups of patients, including children and older people, but there were no data to address these groups specifically.
Values and preferences—Applying the agreed values and preferences (box 2), the guideline panel inferred that most patients would be reluctant to use remdesivir, given the evidence left high uncertainty regarding effects on mortality and the other prioritised outcomes. This was particularly so as any beneficial effects of remdesivir, if they do exist, are likely to be small, and the possibility of important harm remains. The panel acknowledged, however, that values and preferences are likely to vary, and there will be patients and clinicians who choose to use remdesivir given that the evidence has not excluded the possibility of benefit.
Resource implications, feasibility, equity, and human rights—A novel therapy typically requires higher certainty evidence of important benefits than is currently available for remdesivir, preferably supported wherever possible by cost effectiveness analysis. In the absence of this information, the guideline panel raised concerns about opportunity costs and the importance of not drawing attention and resources away from best supportive care or the use of corticosteroids in severe covid-19. It was noted that, currently, remdesivir is administered only by the intravenous route and global availability is limited.
Practical issues—Its use is contraindicated in those with liver dysfunction (ALT >5 times normal at baseline) or renal dysfunction (eGFR <30 mL/minute). To date, it can only be administered intravenously, and it has relatively limited availability.
Corticosteroids (published 4 September 2020)
On 17 July 2020 the guideline panel reviewed evidence from eight RCTs (7184 patients) evaluating systemic corticosteroids versus usual care in treatment of covid-19, seven of which reported mortality data by subgroup of illness severity. Mortality data from one trial, GLUCOCOVID, were not incorporated in the summary of finding for mortality because the mortality outcome data were not available by subgroup. The panel did not consider transdermal or inhaled administration of corticosteroids, high dose or long term regimens, or prophylaxis. The panel did not reach consensus on recommendation 1, which required a vote. The second recommendation was made by consensus.
Whereas the recommendations remain unchanged, the evidence summary available via MAGICapp for corticosteroids was updated before the fifth iteration of the living guideline. The baseline risk estimates for mortality are now based on the WHO SOLIDARITY trial (as for other drugs in this guideline)5 rather than the initial ISARIC cohort study that likely overestimates current mortality risks at the global level.16 This update was also needed to inform the baseline risk for mortality in the evidence summary informing the strong recommendation for IL-6 inhibitors in addition to standard care for patients with severe or critical covid-19, where corticosteroids provide a relative reduction in mortality by 21%.
Understanding the recommendations on corticosteroids
Recommendation 1: We recommend systemic corticosteroids rather than no systemic corticosteroids for the treatment of patients with severe and critical covid-19 (strong recommendation)
Who does it apply to? This recommendation applies to patients with severe and critical covid-19. The panel judged that all or almost all fully informed patients with severe covid-19 would choose to take systemic corticosteroids. The recommendation should apply to patients with severe and critical covid-19 even if they cannot be hospitalised or receive oxygen because of resource limitations.
The applicability of the recommendation is less clear for populations that were under-represented in the considered trials, such as children, patients with tuberculosis, and those who are immunocompromised. In considering potential contraindications to short term systemic corticosteroids in such patients, clinicians must determine if they warrant depriving a patient of a potentially lifesaving therapy. Clinicians should exercise caution in use of corticosteroids in patients with diabetes or underlying immunocompromise. The panel was confident that clinicians using these guidelines would be aware of additional potential side effects and contraindications to systemic corticosteroid therapy, which may vary geographically in function of endemic microbiological flora.
Balance of benefit and harm—Ultimately, the panel made its recommendation on the basis of the moderate certainty evidence of a 28-day mortality reduction of 3.4% in severe and critical covid-19 combined. Systemic corticosteroids compared with no corticosteroid therapy probably reduce the risk of 28-day mortality in these patients (moderate certainty evidence; relative risk 0.79 (95% confidence interval 0.70 to 0.90); absolute effect estimate 34 fewer deaths per 1000 patients (95% CI 48 fewer to 16 fewer)). Therapy also probably reduces the need for mechanical ventilation (moderate certainty evidence, relative risk 0.74 (0.59 to 0.930; absolute effect estimate 30 fewer cases per 1000 patients (48 fewer to 8 fewer)). The effects of systemic corticosteroids on other outcomes are described in the summary of findings.
Overall, the panel has high certainty that the adverse effects when considered together are sufficiently limited in importance and frequency and suggested that corticosteroids administered in these doses for 7-10 days are not associated with an increased risk of adverse events, beyond likely increasing the incidence of hyperglycaemia (moderate certainty evidence; absolute effect estimate 46 more per 1000 patients (23 more to 72 more)) and hypernatraemia (moderate certainty evidence; 26 more per 1000 patients (13 more to 41 more)). In contrast with new agents proposed for covid-19, clinicians have a vast experience of systemic corticosteroids, and the panel was reassured by their overall safety profile.
Values and preferences—The panel took an individual patient perspective to values and preferences but, given the burden of the pandemic for healthcare systems globally, also placed a high value on resource allocation and equity. The benefits of corticosteroids on mortality was deemed of critical importance to patients, with little or no anticipated variability in their preference to be offered treatment if severely ill from covid-19.
Resource implications, feasibility, equity, and human rights—Systemic corticosteroids are low cost, easy to administer, and readily available globally. Dexamethasone and prednisolone are among the most commonly listed medicines in national essential medicines lists; listed by 95% of countries. Accordingly, systemic corticosteroids are among a relatively small number of interventions for covid-19 that have the potential to reduce inequities and improve equity in health. Those considerations influenced the strength of this recommendation.
Acceptability—The ease of administration, the relatively short duration of a course of systemic corticosteroid therapy, and the generally benign safety profile of systemic corticosteroids administered for up to 7-10 days led the panel to conclude that the acceptability of this intervention was high.
Recommendation 2: We suggest not to use corticosteroids in the treatment of patients with non-severe covid-19 (weak or conditional recommendation)
Who does it apply to? This recommendation applies to patients with non-severe disease regardless of their hospitalisation status. The guideline panel noted that patients with non-severe covid-19 would not normally require acute care in hospital or respiratory support, but in some jurisdictions these patients may be hospitalised for isolation purposes only, in which case they should not be treated with systemic corticosteroids. Several specific circumstances were considered.
-
Systemic corticosteroids should not be stopped for patients with non-severe covid-19 who are already treated with systemic corticosteroids for other reasons (such as patients with chronic obstructive pulmonary disease or chronic autoimmune disease).
-
If the clinical condition of patients with non-severe covid-19 worsens (that is, increase in respiratory rate, signs of respiratory distress or hypoxaemia) they should receive systemic corticosteroids (see recommendation 1).
-
Pregnancy: antenatal corticosteroid therapy may be administered for pregnant women at risk of preterm birth from 24 to 34 weeks’ gestation when there is no clinical evidence of maternal infection and adequate childbirth and newborn care are available. In cases where the woman presents with mild or moderate covid-19, the clinical benefits of antenatal corticosteroid might outweigh the risks of potential harm to the mother. In this situation, the balance of benefits and harms for the woman and the preterm newborn should be discussed with the woman to ensure an informed decision, as this assessment may vary depending on the woman’s clinical condition, her wishes and those of her family, and available healthcare resources.
-
Endemic infections that may worsen with corticosteroids should be considered. For example, for Strongyloides stercoralis hyperinfection associated with corticosteroid therapy, diagnosis or empiric treatment may be considered in endemic areas if steroids are used.
Balance of benefit and harm—Systemic corticosteroids may increase the risk of 28-day mortality (low certainty evidence; relative risk 1.22 (95% CI 0.93 to 1.61); absolute effect estimate 39 more per 1000 patients (95% CI 12 fewer to 107 more)). The certainty of the evidence for this specific subgroup was downgraded due to serious imprecision (that is, the evidence does not allow to rule out a mortality reduction) and risk of bias due to lack of blinding. The effects of systemic corticosteroids on other outcomes are described in the summary of findings (infographic and links to MAGICapp).
Values and preferences—The weak or conditional recommendation was driven by likely variation in patient values and preferences. The panel judged that most individuals with non-severe illness would decline systemic corticosteroids. However, many may want them after shared decision making with their treating physician.
Resource implications, feasibility, equity, and human rights—To help guarantee access to systemic corticosteroids for patients with severe and critical covid-19, it is reasonable to avoid their administration to patients who, given the current evidence, do not seem to derive any benefit from this intervention
Uncertainties, emerging evidence, and future research
The guideline recommendations for covid-19 therapeutics demonstrate remaining uncertainties concerning treatment effects for all outcomes of importance to patients. There is also a need for better evidence on prognosis and values and preferences of patients with covid-19.
Here we outline key uncertainties for IL-6 receptor blockers identified by the guideline panel, adding to those for corticosteroids in the first version, remdesivir in the second version, hydroxychloroquine and lopinavir-ritonavir in the third version, and ivermectin in the fourth version of the living guideline. These uncertainties may inform future research—that is, the production of more relevant and reliable evidence to inform policy and practice. We also outline emerging evidence in the rapidly changing landscape of trials for covid-19.
IL-6 receptor blockers
Despite the strong recommendation, some uncertainties persist:
-
Long term mortality and functional outcomes in covid-19 survivors
• Long term safety data in terms of nosocomial infections
• Data in children, pregnant patients, and those who are already immunocompromised
• Patients with non-severe covid-19
• Immunity and the risk of a subsequent infection, which may affect the risk of death after 28 days
• Outcomes by different IL-6 receptor blocker dosing and optimal timing of drug initiation.
Ivermectin
Given the very low certainty in estimates for most critical outcomes of interest, the guideline panel felt that further high quality clinical trials examining this drug would be essential before any recommendation for use as part of clinical care. This includes further RCTs examining both inpatients and outpatients, patients with varying disease severities, and using different ivermectin dosing regimens. The focus of these studies should be on outcomes important to patients such as mortality, quality of life, need for hospitalisation, need for invasive mechanical ventilation, and time to clinical or symptom improvement. Also, a better characterisation of potential harms with ivermectin in patients with covid-19 is important.
Hydroxychloroquine and lopinavir-ritonavir
Although some uncertainty remains, the guideline panel felt that further research was unlikely to uncover a subgroup of patients who would benefit from hydroxychloroquine or lopinavir-ritonavir on the most important outcomes (mortality, mechanical ventilation) given the consistent results in trials across disease severity and location.
Remdesivir
Remaining uncertainties include effects on:
-
Critical outcomes of interest, particularly those that impact resource allocation, such as the need for mechanical ventilation, duration of mechanical ventilation, and duration of hospitalisation
-
Specific subgroups, such as different severities of illness, different time (days) since onset of illness, children and older adults, pregnant women, duration of therapy
-
Long term outcomes (such as 1-year endpoint) examining mortality or long term quality of life
-
Long term safety and rare but important side effects
-
Patient-reported outcomes such as symptom burden
-
Outcomes when used in combination with other agents such as, but not limited to, corticosteroids
-
Impact on viral shedding, viral clearance, patient infectivity.
Corticosteroids
Remaining uncertainties include effects on:
-
Long term mortality and functional outcomes in covid-19 survivors
-
Patients with non-severe covid-19 (that is, pneumonia without hypoxaemia)
-
When used in combination with additional therapies for covid-19, such as novel immunomodulators. It will become increasingly important to ascertain how these interact with systemic corticosteroids. All investigational therapies for severe and critical covid-19 (including remdesivir) should be compared with systemic corticosteroids or evaluated in combination with systemic corticosteroids versus systemic corticosteroids alone
-
Immunity and the risk of a subsequent infection, which may affect the risk of death after 28 days
-
By different steroid preparation, dosing, and optimal timing of drug initiation.
Emerging evidence
The unprecedented volume of planned and ongoing studies for covid-19 interventions—over 3000 RCTs as of 1 March 2021—implies that more reliable and relevant evidence will emerge to inform policy and practice.2 An overview of registered and ongoing trials for covid-19 therapeutics is available from the Infectious Diseases Data Observatory, through their living systematic review of covid-19 clinical trial registrations2 and WHO website https://www.covid-nma.com/dataviz/.
Although most of these studies are small and of variable methodological quality, some large, international platform trials (such as RECOVERY and SOLIDARITY) are better equipped to provide robust evidence for several potential treatment options. Such trials can also adapt their design, recruitment strategies, and selection of interventions based on new insights.
Concerning tocilizumab and sarilumab and covid-19, more than 73 RCTs planning to enrol more than 36 730 participants (range 24-12 000) are registered or ongoing. Regarding tocilizumab, 58 RCTs planning to enrol 33 189 participants (range 24-12000) are now registered or ongoing. Regarding sarilumab, 13 RCTs planning to enrol 12 251 participants (range: 27-7100) are now registered and ongoing (see appendix 6).
How patients were involved in the creation of this article
The guideline panel included four patients who have had covid-19. Their perspectives were crucial in considering the values and preferences associated with IL-6 receptor blockers, ivermectin, hydroxychloroquine, lopinavir-ritonavir, remdesivir, and corticosteroids.
Acknowledgments
We thank all the following collaborators who contributed to this endeavour, as detailed in the WHO guidance (see link in box 1)
• World Health Organisation (WHO) Secretariat for Therapeutics and COVID-19
• WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group for their publication: Association of Administration of Interleukin-6 Antagonists with Mortality and Other Outcomes Among Hospitalized Patients With COVID-19: A Prospective Meta-Analysis.
• External reviewers for WHO
We also thank
• The living systematic review and network meta-analysis team, led by investigators Reed Siemienuk and Romina Brignardello-Petersen at McMaster University, Canada
• BMJ editorial team of Greg Cotton (technical editor), Navjoyt Ladher (head of education), and Will Stahl-Timmins (data graphics designer) for their work on this living guideline publication in The BMJ
• Brittany Maguire, Philippe Guerin, Lorenzo Arena and Sumayyah Rashan for providing up to date data on ivermectin trials from the Infectious Diseases Data Observatory (IDDO) living systematic review for covid-19 clinical trial registration (https://www.iddo.org/research-themes/covid-19/live-systematic-clinical-trial-review)
• Andrew Owen (University of Liverpool, UK) for contributions to subgroup analysis of hydroxychloroquine by modelling expected serum concentrations over time
Footnotes
-
Funding: No specific funding was provided for this guideline, with MAGIC providing pro-bono contributions and support to WHO in the context of the COVID-19 pandemic.
-
Competing interests: All guideline panel members have completed the WHO interest disclosure form. All authors have completed the BMJ Rapid Recommendations interest of disclosure form. The WHO, MAGIC and The BMJ judged that no panel member had any financial conflict of interest. Professional and academic interests are minimised as much as possible, while maintaining necessary expertise on the panel to make fully informed decisions. MAGIC and TheBMJ assessed declared interests from other co-authors of this publication and found no relevant conflicts of interests.
-
Provenance and peer review: This publication was commissioned by The BMJ in partnership with WHO and the MAGIC Evidence Ecosystem Foundation, in the context of the BMJ Rapid Recommendations. Pre-publication internal and external peer-review managed by WHO, and internal review at The BMJ. Post-publication review through rapid responses on bmj.com and through MAGICapp.
- Bram Rochwerg, methods chair IL-6 receptor blockers, ivermectin, remdesivir and lopinavir-ritonavir, critical care physician12ac,
- Arnav Agarwal, methodologist, internist123*,
- Reed AC Siemieniuk, methods chair hydroxychloroquine12a*,
- Thomas Agoritsas, internist, methodologist145*b,
- François Lamontagne, methods chair corticosteroids, critical care physician6*b,
- Lisa Askie, WHO staff, clinical team for covid-19 response7,
- Lyubov Lytvyn, methodologist1*,
- Yee-Sin Leo, clinical chair corticosteroids, infectious disease specialist8abc,
- Helen Macdonald, UK research editor9*,
- Linan Zeng, methodologist1*,
- Wagdy Amin, chest physician10ac,
- Erlina Burhan, chest physician11ac,
- Frederique Jacquerioz Bausch, infectious disease physician12ac,
- Carolyn S Calfee, intensive care physician13bc,
- Maurizio Cecconi, critical care physician14abc,
- Duncan Chanda, infectious disease physician15ac,
- Bin Du, critical care physician16ac,
- Heike Geduld, emergency physician17abc,
- Patrick Gee, patient panel member18abc,
- Nerina Harley, critical care physician19c,
- Madiha Hashimi, critical care physician20ac,
- Beverly Hunt, haematologist21c,
- Sushil K Kabra, paediatric chest physician22ac,
- Seema Kanda, patient partner23abc,
- Leticia Kawano-Dourado, respiratory medicine physician24abc,
- Yae-Jean Kim, paediatric infectious disease physician25abc,
- Niranjan Kissoon, paediatric critical care physician26abc,
- Arthur Kwizera, critical care physician27abc,
- Imelda Mahaka, patient partner28ac,
- Hela Manai, emergency physician29abc,
- Greta Mino, paediatric infectious disease physician30ac,
- Emmanuel Nsutebu, infectious disease physician31ac,
- Jacobus Preller, WHO staff, clinical team for covid-19 response7,
- Natalia Pshenichnaya, infectious disease physician32ac,
- Nida Qadir, critical care physician33abc,
- Saniya Sabzwari, geriatrician34ac,
- Rohit Sarin, chest physician35abc,
- Manu Shankar-Hari, critical care physician36c,
- Michael Sharland, infectious disease physician37ac,
- Yinzhong Shen, infectious disease physician38abc,
- Shalini Sri Ranganathan, paediatric physician39ac,
- Joao P Souza, obstetrics and gynaecology physician40ac,
- Miriam Stegemann, infectious disease and chest physician41c,
- An De Sutter, public health and primary care physician42c,
- Sebastian Ugarte, intensive care physician43ac,
- Sridhar Venkatapuram, ethicist44ac,
- Vu Quoc Dat, infectious disease physician45ac,
- Dubula Vuyiseka, patient partner46ac,
- Ananda Wijewickrama, infectious disease physician47ac,
- Brittany Maguire, methodologist48*,
- Dena Zeraatkar, methodologist1*,
- Jessica J Bartoszko, methodologist1*,
- Long Ge, methodologist149*,
- Romina Brignardello-Petersen, methodologist1*,
- Andrew Owen, clinical pharmacologist50*,
- Gordon Guyatt, methodologist12*,
- Janet Diaz, WHO lead, clinical team for covid-19 response7*d,
- Michael Jacobs, clinical chair IL-6 receptor blockers, ivermectin, remdesivir, hydroxychloroquine, and lopinavir-ritonavir, infectious disease specialist51acd,
- Per Olav Vandvik, methodologist, internist552*d
- Correspondence to: Bram Rochwerg rochwerg@mcmaster.ca or Michael Jacobs michael.jacobs@ucl.ac.uk
LINK
https://www.bmj.com/content/370/bmj.m3379